Why Does Ice Float in Water?

© Geekswipe. All Rights Reserved.
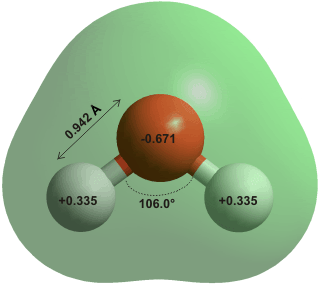
The water molecule as we know, is made up of two hydrogen atoms, which are covalently bonded to one oxygen atom (polar covalent bonds). Further, the water molecule itself is bonded with another neighboring water molecule by hydrogen bonding, which is due to the polarity of the water molecule. The polarity is because of the fact that the oxygen atom is more electronegative, compared to Hydrogen atoms. The oxygen atom tends to attract electrons stronger than the poor hydrogen atoms, which get attracted themselves. When an oxygen atom (relatively negative polarity) in a water molecule attracts the electrons of a neighboring molecule and the hydrogen atom (with a relatively positive polarity) of those molecules, they form hydrogen bonds between the molecules.
Hydrogen bonds in liquid phase vs solid phase
When the water is in liquid form, the kinetic energy of the water molecules is high due to the temperature and this overall high kinetic energy breaks these hydrogen bonds easily. This makes the water molecules to move rapidly, forming and breaking hydrogen bonds very quickly, bumping and nudging each other. But when the temperature is lowered, the kinetic energy lowers so that the hydrogen bonds can be easily formed. The water molecules gradually start forming a regular pattern, due to the hydrogen bonding. Though the water reaches its maximum density when it reaches at 4 °C, upon cooling further to its freezing point at 0 °C, the kinetic energy lowers even further below that it no longer could break any hydrogen bonds. Then the water molecules starts forming a crystal structure with hydrogen bonds holding them farther apart from each other, forming more space in the lattice. Or in simpler words, they expand and occupy more space with lots of holes in between them, increasing the overall volume.
Uh… Holes?
Yeah, holes :) The holes form due to the geometrical structure of the water molecule. Look at the water molecule below.
The common angle between the oxygen atoms (H-O-H angle) is 104.52° to 109.5°. In ice, a single water molecule forms four hydrogen bonds with the neighboring water molecules at this angle—two bonds between the two hydrogen atoms and other two oxygen atoms, other two bonds between other molecules and the two lone pair electrons of the oxygen atom. So when they re-align themselves at the freezing point, they form a beautiful lattice of orderly arranged molecules and with holes in them, taking up more volume and expanding.
Well how this expansion and holes makes the ice to float in the water? Well, for anything to float in a medium, its density should be lower than that of the medium. In this case, when the temperature is lowered below 4 °C, the water molecules expand and create these spaces between them. This increases the volume with much lesser molecules and hence lowers the density of the solid ice by 9%. Hence, the whitey chill ice floats peacefully.
This post was first published on January 2, 2015.