Tagged: Chemical Bonds, Organic Chemistry
Are there any specific methods defined for calculating the angles? How did they calculate the bond angles in earlier times?
Sorry for a very delayed response. Curiosity is actually growing and we don’t have a lot of members yet. Thanks for being patient and not bumping the thread. :) Anyways, I’ll give you a basic answer.
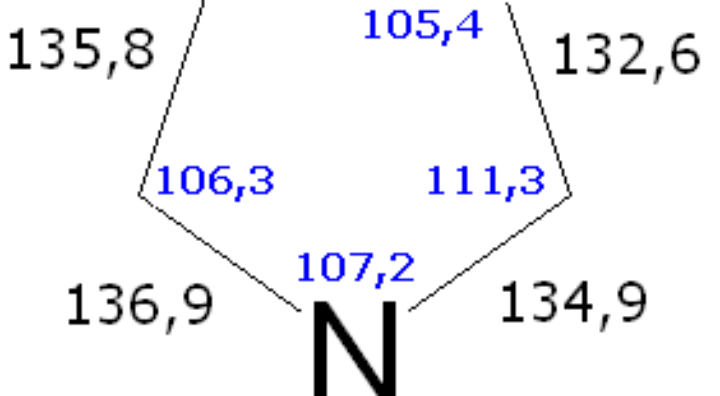
Determination of the bond-angle mainly involves spectroscopy where the molecules absorb electromagnetic radiation and vibrates in a different state than normal. By analyzing the spectra, chemists determine the angles. The other technique used is VSEPR. A simple definition of this theory is that it works under the assumption that the atoms in any molecule will arrange themselves to a certain geometrical structure so that the repulsion between them is kept to a minimum.
But as far as I know, the common method used to find the structure of a crystal lattice, is x-ray crystallography. With this method, the molecular structure of a crystal can be rendered from the diffraction pattern caused by passing the x-rays through the crystal. The method used to determine the bond angles is precisely called as single crystal crystallography.
In addition to these models, complex molecules would involve computational determinations usings simulations using fourier transforms.
I am really not that into chemistry, but I guess this will help you to start reading some in-depth explanations on Wikipedia or other articles while you wait for a professional answer from someone who is really into organic chemistry.
the atoms in any molecule will arrange themselves to a certain geometrical structure
It is still a prediction! I researched some other forums, and it turns out that there is no numerical approach to this.
You must be logged in to reply to this topic. Log In
