In my school, I was taught that neutrons have no charge at all. My friend says otherwise! He says that the neutrons have both positive and negative charge that add to zero. Is he right?
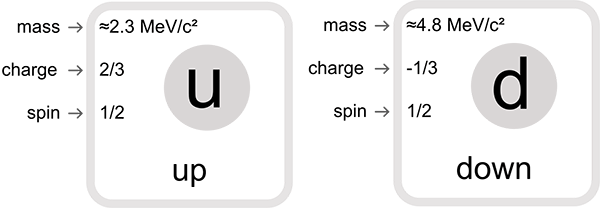
Your friend is absolutely right. I think he might have explained it further. I will put it close to the layman terms here. Neutrons are made up of three fundamental particle known as quarks. Two down quarks and one up quark (two stable form of quarks). When the charges of these three quarks are added up, one will get the net charge as zero. Which proves that the neutrons have zero ‘net’ charge. Add it up and check for yourself.

It is worth noting that neutrons have a very negligible amount of negative charge of −3×10−41 C.
Sorry it has been a day. Away for weekend! Thank you for the answers guys. While I read more about this I bumped onto the fact that hydrogen doesn’t have a neutron. Aren’t all atoms supposed to have protons and neutrons in their nucleus?
Welcome to Geekswipe SharpLuke.
Yes, all atoms are supposed to have protons and neutrons together in their nucleus, except for hydrogen. After the big bang, the universe was filled with only the primordial nucleons consisting of protons and neutrons. Lucky for protons, the free neutrons were unstable as they decayed into protons mostly. So all that was left to form into a nucleus was the proton itself on its own. When electrons and protons mutually attracted each other, they formed the hydrogen atom. So it simply is that the hydrogen atom (protium) didn’t need a neutron. But when they fused to form helium and other heavier elements, things got interesting as the neutrons served the purpose of holding multiple protons by overcoming the mutual repulsion of the protons with the combined strong nuclear force of neutrons themselves. If you are still wondering, there are hydrogen isotopes with neutrons in it too. Deuterium and Tritium. Worth exploring, if this is your first time getting to know these stuff.
I have one more question. Why doesn’t the electron in Hydrogen atom collapse into proton? Is it because of the Heisenberg’s uncertainty principle?
Spot on! :)
Edit: Hey @sharpluke, if you are still interested, here are some more info about this – Uncertainty Principle and Atomic Nucleus.
The question should not have raised in you if you have understood the uncertainty principle. Read about ‘orbitals’ and you will find yourself answering your question in an enlightening way.
Sorry again for replying after a long time. I am bothered by neutron stars now. If neutrons are unstable, how do they exist in the neutron stars?
You mean ‘how do they exist in neutron stars without decaying?’ If yes, the answer is gravity! Gravity makes it impossible for all those neutrons to decay into protons and electrons. Think of the neutron star as a big nucleus of an element. When enough neutrons decay into electrons and protons, Pauli’s exclusion principle disallows any further states of the electrons, protons or neutrons. This makes weird stuffs to the neutrons in the neutron star, forming the degenerate neutron gas. Further decay and their ‘existence’ is preserved by the pressure created under the exclusion principle, called as neutron degeneracy pressure.
I understood something. FWIW I learned something new about degenerate matter. Thank you for taking time in explain me things.
You must be logged in to reply to this topic. Log In