Curiosity › Science › Physics › How ice turns into water vapor without melting to water first? › Reply To: How ice turns into water vapor without melting to water first?
Substances change phase when there is a change in its energy. The usual process we are used to at home is water boils to form water vapor and freezes to form ice. This gives the idea that water is an intermediary liquid state between gas and solid. This is right! But not all the times.
Under certain atmospheric conditions, a solid like ice can change its phase to a gaseous phase and vice versa without going through the intermediary liquid phase. Ice changing from solid phase to vapor is known as sublimation. Similarly, vapor changing to solid ice is known as deposition.
This is the phase diagram for water:
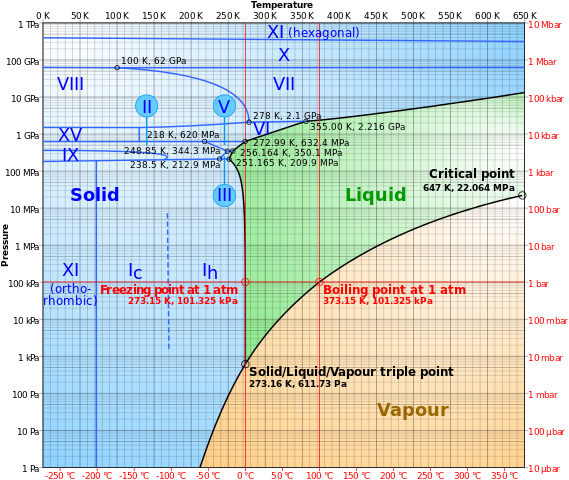
When the atmospheric condition meet the right conditions where the temperature is below 0 and the pressure is lower than 10 kPa, the solid form of water (ice) will undergo sublimation to form gas (water vapor). You could see this atop peaks like Everest.
