Yes, it can. They can homogeneously change between one phase to the other at a particular temperature and pressure or exist as a. Thermodynamically, this particular temperature and pressure, at which a matter occur at all the three phases (solid, liquid, and gas), is known as the triple point. The following is the phase diagram of the water showing the triple point of water 273.16 K.
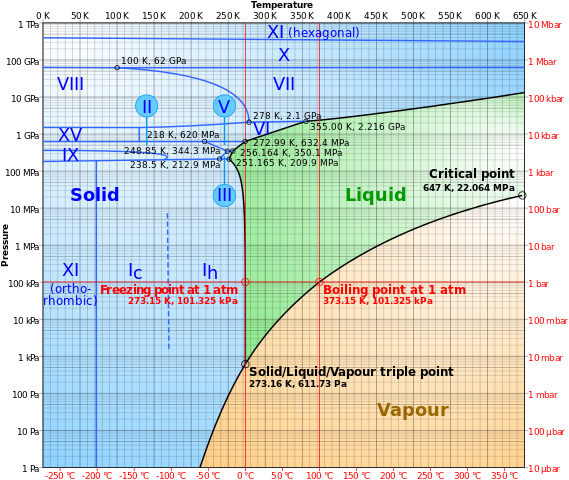
Illustration by Cmglee | CC BY-SA
So at 273.16 K and 611.65 Pa, water exists as a homogenous mixture of solid, liquid, and gaseous phases, where any minimal changes to the temperature or pressure of the system can force a rapid transition between these phases.

